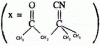Q. 1 – Q. 45 carry one mark each, for which only one option is correct. Any wrong answer will lead to deduction of 1/3 mark.
1. In diborane, the number of electrons that account for bonding in the bridges is
(A) six (B) two (C) eight (D) four
2. The optically active molecule is
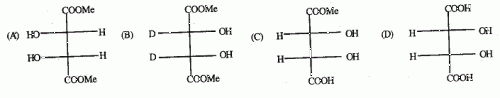
Ans is : C
3. A van der Waals gas may behave ideally when
(A) The volume is very low
(B) The temperature is vey high
(C) The pressure is vey low
(D) The temperature, pressure and volume all are very high
4. The half-life for decay of 14C by β-emission is 5730 years. The fraction of 14C decays, in a sample that is 22,920 years old, would be
(A) 1/8 (B) 1/16 (C) 7/8 (D) 15/16
5. 2-Methylepropane on monochlorination under photochemical condition give
(A) 2-Chloro-2-methylepropane as major product
(B) (1:1) Mixture of 1-chloro-2-methylepropane and 2-chloro-2-methylepropane
(C) 1-Chloro-2-methylepropane as major product
(D) (1:9) Mixture of 1-chloro-2-methylepropane and 2-chloro-2-methylepropane
6. For a chemical reaction at 27°C, the activation energy is 600 R. The ratio of the rate constants at 327°C to that of at 27°C will be
(A) 2 (B) 40 (C) e (D) e²
7. Chlorine gas reacts with red hot calcium oxide to give
(A) Bleaching powder and dichlorine monoxide
(B) Bleaching powder and water
(C) Calcium chloride and chlorine dioxide
(D) Calcium chloride and oxygen
8. Correct pair of compounds which gives blue colouration/precipitate and white precipitate, respectively, when their Lassaigne's test is separately done is
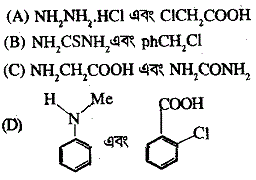
Ans is : (D)
9. The change of entropy (dS) is defined as
(A) dS=δq/T (B) dS = dH/T (C) dS=δqeqv/T (D) dS = (dH-dG)/T
10. In O2 and H2O2, the O-O bond lengths are 1.21 and 1.48 Å respectively. In ozone, the average O-O bond length is
(A) 1.28 Å (B) 1.18 Å (C) 1.44 Å (D) 1.52 Å
11. The IUPAC name of the compound X is
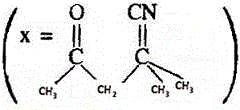
(A) 4-cyano-4-methyle-2-oxopentane
(B) 2-cyano-2-methyle-4-oxopentane
(C) 2,2-dimethyle-4-oxopentanenitrile
(D) 4-cyano-4-methyle-2-pentanone
12. At 25°C, the solubility product of a salt of MX2 type is 3.2 x 10-8 in water. The solubility (in moles/lit) of MX2 in water at the same temperature will be
(A) 1.2 x 10-3 (B) 2 x 10-3 (C) 3.2 x 10-3 (D) 1.75 x 10-3
13. In SOCl2, the Cl-S-Cl and Cl-S-O bond angles are
(A) 130° and 115° (B) 106° and 96° (C) 107° and 108° (D) 96° and 106°
14. (+)-2-chloro-2-phenylethane in toluene racemises slowly in the presence of small amount of SbCl5, due to the formation of
(A) carbanion (B) carbene (C) free-radical (D) carbocation
15. Acid catalysed hydrolysis of ethyle acetate follows a pseudo-first order kinetics with respect to ester. If the reaction is carried out with large excess of ester, the order with respect to ester will be
(A) 1.5 (B) 0 (C) 2 (D) 1
16. The different colours of litmus in acidic, neutral and basic solutions are, respectively,
(A) Red, orange and blue
(B) Blue, violet and red
(C) Red, colourless and blue
(D) Red, violet and blue
17. Baeyer's reagent is
(A) Alkaline potassium permanganate
(B) Acidified potassium permanganate
(C) Neutral potassium permanganate
(D) Alkaline potassium manganate
18. The correct order of equivalent conductances at infinite dilution in water at room temperature for
H+,K+,CH3COO− and HO− ions is
(A) HO−>H+>K+>CH3COO−
(B) H+>HO−>K+>CH3COO−
(C) H+>K+>HO−>CH3COO−
(D) H+>K+>CH3COO−>HO−
19. Nitric acid can be obtained from ammonia via the formations of the intermediate compounds
(A) Nitric oxides and nitrogen dioxides
(B) Nitrogen and nitric oxides
(C) Nitric oxide and dinitrogen pentoxide
(D) Nitrogen and nitrous oxide
20. In the following species, the one which is likely to be the intermediate during benzoin condensation of benzaldehyde, is
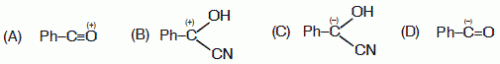
Ans is : (C)
21. The correct order of acid strength of the following substituted phenols in water at 28°C is
(A) p-nitrophenol < p-fluorophenol < p-chlorophenol
(B) p-chlorophenol < p-fluorophenol < p-nitrophenol
(C) p-fluorophenol < p-chlorophenol < p-nitrophenol
(D) p-fluorophenol < p-nitrophenol < p-chlorophenol
22. For isothermal expansion of an ideal gas, the correct combination of the thermodynamic parameters will be
(A) ΔU = 0, Q = 0, w ≠ 0 and ΔH ≠ 0
(B) ΔU ≠ 0, Q ≠ 0, w ≠ 0 and ΔH ≠ 0
(C) ΔU = 0, Q ≠ 0, w = 0 and ΔH ≠ 0
(D) ΔU = 0, Q ≠ 0, w ≠ 0 and ΔH ≠ 0
23. Addition of excess potassium iodide solution to a solution of mercuric chloride gives the halide complex
(A) tetrahedral K2[HgI4]
(B) trigonal K[HgI3]
(C) linear Hg2I2
(D) square planar K2[HgCl2I2]
24. Amongst the following, the one which can exist in free state as a stable compound is
(A) C7H9O (B) C8H12O (C) C6H11O (D) C10H17O2
25. A conductivity cell has been calibrated with a 0.01 M 1:1 electrolyte solution (specific conductances k=1.25 x 10-3 S cm-1) in the cell and the measured resistance was 800 ohms at 25°C. The constant will be
(A) 1.02 cm-1 (B) 0.102 cm-1 (C) 1.00 cm-1 (D) 0.5 cm-1
26. The orange solid on heating gives a colourless gas and a green solid which can be reduced to metal by aluminium powder. The orange and the green solids are, respectively
(A) (NH4)2Cr2O7 and Cr2O3
(B) Na2Cr2O7 and Cr2O3
(C) K2Cr2O7 and CrO3
(D) (NH4)2Cr2O4 and CrO3
27. The best method for the preparation of 2,2-dimethylbutane is via the reaction of
(A) Me3CBr and MeCH2Br in Na/ether
(B) (Me3C)2CuLi and MeCH2Br
(C) (MeCH2)2CuLi and Me3CBr
(D) Me3CMgI and MeCH2I
28. The condition of spontaneity of process is
(A) lowering of entropy at constant temperature and pressure
(B) lowering of Gibbs free energy of system at constant temperature and pressure
(C) increase of entropy of system at constant temperature and pressure
(D) increase of Gibbs free energy of the universe at constant temperature and pressure
29. The increasing order of O-N-O bond angle in the species NO2,NO+2 and NO−2 is
(A) NO+2<NO2<NO−2
(B) NO2<NO−2<NO+2
(C) NO+2<NO−2<NO2
(D) NO2<NO+2<NO−2
Ans : No option
30. The correct structure of the dipeptide gly-ala is
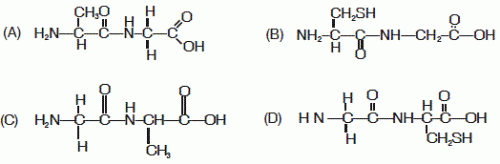
Ans is : (C)
31. Equivalent conductivity at infinite dilution for sodium-potassium oxalate ((COO−)2Na+K+) will be [given, molar conductivities of oxalate, K+ and Na+ ions at infinite dilution are 148.2, 50.1, 73.5 S cm²mol-1, respectively]
(A) 271.8 S cm²eq-1 (B) 67.95 S cm²eq-1 (C) 543.6 S cm²eq-1 (D) 135.9 S cm²eq-1
32. For BCl3,AlCl3 and GaCl3 the increasing order of ionic character is
(A) BCl3<AlCl3<GaCl3
(B) GaCl3<AlCl3<BCl3
(C) BCl3<GaCl3<AlCl3
(D) AlCl3<BCl3<GaCl3
33. At 25°C, pH of a 10-8 M aqueous KOH solution will be
(A) 6.0 (B) 7.02 (C) 8.02 (D) 9.02
34. The reaction of nitroprusside anion with sulphide ion gives purple colouration due to the formation of
(A) the tetranionic complex of iron(II) coordinating to one NOS− ion
(B) the dianionic complex of iron(II) coordinating to one NCS− ion
(C) the trianionic complex of iron(III) coordinating to one NOS− ion
(D) the tetranionic complex of iron(III) coordinating to one NCS− ion
35. An optically active compound having molecular formula C8H16 on ozonolysis gives acetone as one of the products. The structure of the compound is
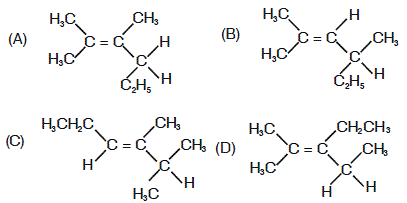
Ans is : B
36. Mixing of two different ideal gases under isothermal reversible condition will lead to
(A) increase of Gibbs free energy of the system
(B) no change of entropy of the system
(C) increase of entropy of the system
(D) increase of enthalpy of the system
37. The ground state electronic configuration of CO molecule is
(A) 1σ22σ21π43σ2
(B) 1σ22σ23σ21π22π2
(C) 1σ22σ21π23σ22π2
(D) 1σ21π42σ23σ2
38. When aniline is nitrated with nitrating mixture in ice cold condition, the major product obtained is
(A) p-nitroaniline (B) 2,4-dinitroaniline (C) o-notroaniline (D) m-nitroaniline
39. The measured freezing point depression for a 0.1 m aqueous CH3COOH solution is 19 °C. The acid dissiociation constant Ka at the concentration will be (Given Kf, the molal cryoscopic constant = 1.86 K kg mol-1)
(A) 4.76 x 10-5 (B) 4 x 10-5 (C) 8 x 10-5 (D) 2 x 10-5
40. The ore chromite is
(A) FeCr2O4 (B) CoCr2O3 (C) CrFe2O4 (D) FeCr2O3
41. 'Sulphan' is
(A) a mixture of SO3 and H2SO5
(B) 100% conc. H2SO4
(C) a mixture of gupsum and conc. H2SO4
(D) 100% oleum (a mixture of 100% SO3 in 100% H2SO4)
42. Pressure-volume (PV) work done by an ideal gaseous system at constant volume is (where E is internal energy of the system)
(A) -ΔP/P (B) zero (C) -VΔP (D) -ΔE
43. Amongst [NiCl4]2−, [Ni(H2O)6]2+, [Ni(PPh3)2Cl2], [Ni(CO)4] and
[Ni(CN)4]2−, the paramagnetic species are
(A) [NiCl4]2−, [Ni(H2O)6]2+, [Ni(PPh3)2Cl2]
(B) [Ni(CO)4], [Ni(PPh3)2Cl2], [NiCl4]2−
(C) [Ni(CN)4]2−, [Ni(H2O)6]2+, [NiCl4]2−
(D) [Ni(PPh3)2Cl2], [Ni(CO)4], [Ni(CN)4]2−
44. Number of hydrogen ions present in 10 millionth part of 1.33 cm³ of pure water at 25°C is
(A) 6.023 million (B) 60 million (C) 8.01 million (D) 80.23 million
45. Ribose and 2-deoxyribose can be differentiated by
(A) Fehling's reagent (B) Tollens's reagent (C) Barfoed's reagent (D) Osazone formation
Q. 46 – Q. 55 carry two marks each, for which only one option is correct. Any wrong answer will lead to deduction of 2/3 mark
46. The standard Gibbs free energy change (ΔG0) at 25°C for the dissociation of N2O4(g) to NO2(g) is (given, equilibrium constant = 0.15, R = 8.314 JK/mol)
(A) 1.1 kj (B) 4.7 kj (C) 8.1 kj (D) 38.2 kj
47. Bromination of PhCOMe in acetic acid medium produces mainly
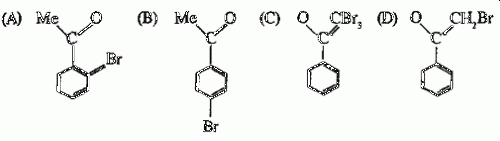
Ans is : D
48. Silicone oil is obtained from the hydrolysis and polymerisation of
(A) trimethylchlorosilane and dimethyldichlorosilane
(B) trimethylchlorosilane and methyldichlorosilane
(C) methylchlorosilane and dimethyldichlorosilane
(D) triethylchlorosilane and diethyldichlorosilane
49. Tratment of 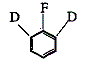 with NaNH2/liq.NH3 gives
with NaNH2/liq.NH3 gives
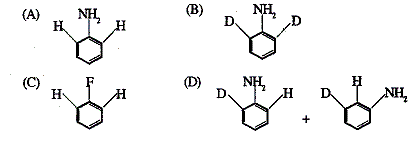
Ans is : D
50. Identify the CORRECT statement
(A) Quantum numbers (n, l, m, s) are obtained arbitrarily
(B) All the quantum numbers (n, l, m, s) for any pair of electrons in an atom can be identical under special circumstance
(C) All the quantum numbers (n, l, m, s) may not be required to describe an electron of an atom completely
(D) All the quantum numbers (n, l, m, s) are required to describe an electron of an atom completely
51. In borax the number of B-O-B links and B-OH bonds present are, respectively,
(A) Five and four (B) Four and five (C) Three and four (D) Five and five
52. Reaction of benzene with Me3COCl in the presence of anhydrous AlCl3 gives
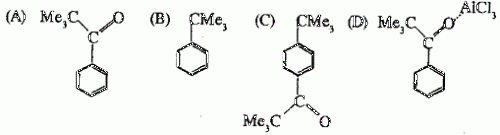
Ans is : B
53. 1 x 10-3 mole of HCl is added to a buffer solution made up of 0.01M acetic acid and 0.01M sodium acetate. The final pH of the buffer will be (given, pKa of acetic acid is 4.75 at 25°C)
(A) 4.60 (B) 4.66 (C) 4.75 (D) 4.8
54. The best method for preparation of Me3CCN is
(A) To react Me3COH with HCN
(B) To react Me3CBr with NaCN
(C) To react Me3CMgBr with ClCN
(D) To react Me3CLi with NH2CN
55. On heating, chloric acid decompose to
(A) HClO4,Cl2,O2 and H2O
(B) HClO2,Cl2,O2 and H2O
(C) HClO,Cl2O and H2O2
(D) HCl,HClO,Cl2O and H2O
Q. 56 – Q. 60 carry two marks each, for which one or more than one options may be correct. Marking of correct options will lead to a maximum mark of two on pro rata basis. There will be no negative marking for these questions. However, any marking of wrong option will lead to award of zero mark against the respective question-irrespective of the number of correct options marked.
56. Consider the following reaction for 2NO2(g)+F2(g)→2NO2F(g). The expression for the rate of reaction in terms of the rate of change of partial pressure of reactant and product is/are
(A) rate = −12[dp(NO2)/dt]
(B) rate = 12[dp(NO2)/dt]
(C) rate = −12[dp(NO2F)/dt]
(D) rate = 12[dp(NO2F)/dt]
57. Tautomerism is exhibited by
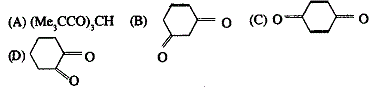
Ans is : A
58. The important advantage(s) of Lintz and Donawitz (L.D) process for the manufacture of steel is/are
(A) The process is very quick
(B) Operating costs are low
(C) Better quality steel is obtained
(D) Scrap iron can be used
59. In basic medium the amount of Ni2+ in a solution can be estimated with the dimethylglyoxime reagent. The correct statement(s) about the reaction and the product is/are
(A) In ammoniacal solution Ni2+ salts give cherry-red precipitate of nickel (II) dimethylglyoximate
(B) Two dimethylglyoximate units are bound to one Ni2+
(C) In the complex two dimethylglyoximate units are hydrogen bonded to each other
(D) Each dimethylglyoximate unit forms a six-membered chelate ring with Ni2+
60. Correct statement(s) in cases of n-butanol and t-butanol is/are
(A) Both are having equal solubility in water
(B) t-butanol is more soluble in water than n-butanol
(C) Boiling point of t-butanol is lower than n-butanol
(D) Boiling point of n-butanol is lower than t-butanol
***