Subject: Physics & Chemistry
Duration : Two Hours Maximum Marks : 100
Subject: Physics
Q.1 - Q.30 carry one mark each.
1. A train approaching a railway platform with a speed of 20 ms-1 starts blowing the whistle. Speed of sound in air is 340ms-1. If the frequency of the emitted sound from the whistle is 640 Hz, the frequency of sound to a person standing on the platform will appear to be
(A) 600 Hz (B) 640 Hz (C) 680 Hz (D) 720 Hz
2. A straight wire of length 2 m carries a current of 10 A. If this wire is placed in a uniform magnetic field of 0.15 T making an angle of 45°with the magnetic field, the applied force on the wire will be
(A) 1.5 N (B) 3 N (C) (D)
3. What is the phase difference between two simple harmonic motions represented by
and
(A) (B)
(C)
(D)
4. Heat is produced at a rate given by H in a resistor when it is connected across a supply of voltage V. If now the resistance of the resistor is doubled and the supply voltage is made V/3 then the rate of production of heat in the resistor will be
(A) H/18 (B) H/9 (C) 6H (D) 18H
5. Two elements A and B with atomic numbers and
are used to produce characteristic x-rays with frequencies
and
respectively. If
, then
will be
(A) (B) 1 : 8 (C) 4 : 1 (D) 1 : 4
6. The de Broglie wavelength of an electron moving with a velocity c/2 (c = velocity of light in vacuum) is equal to the wavelength of a photon. The ratio of the kinetic energies of electron and photon is
(A) 1 : 4 (B) 1 : 2 (C) 1 : 1 (D) 2 : 1
7. Two infinite parallel metal planes, contain electric charges with charge densities +σ and -σ respectively and they are separated by a small distance in air. If the permittivity of air is Ɛo then the magnitude of the field between the two planes with its direction will be
(A) σ/Ɛo towards the positively charged plane
(B) σ/Ɛo towards the negatively charged plane
(C) σ/(2Ɛo) towards the positively charged plane
(D) 0 and towards any direction
8. A box of mass 2 kg is placed on the roof of a car. The box would remain stationary until the car attains a maximum acceleration. Coefficient of static friction between the box and the roof of the car is 0.2 and g = 10 ms-2 . This maximum acceleration of the car, for the box to remain stationary, is
(A) 8 ms-2 (B) 6 ms-2 (C) 4 ms-2 (D) 2 ms-2
9. The decimal number equivalent to a binary number 1011001 is
(A) 13 (B) 17 (C) 89 (D) 178
10. The frequency of the first overtone of a closed pipe of length l1 , is equal to that of the first overtone of an open pipe of length l2 . The ratio of their lengths (l1 : l2) is
(A) 2 : 3 (B) 4 : 5 (C) 3 : 5 (D) 3 : 4
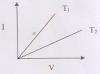
11. The l-V characteristics of a metal wire at two different
temperatures (T1 and T2) are given in the adjoining figure.
Here, we can conclude that
(A) T1 > T2 (B) T1 < T2 (C) T1 = T2 (D) T1 = 2T2
12. In a slide calipers, (m+1) number of vernier divisions is equal to m number of smallest main scale divisions. If d unit is the magnitude of the smallest main scale division, then the magnitude of the vernier constant is
(A) d/(m+1) unit (B) d/m unit (C) md/(m+1) unit (D) (m+1)d/m unit
13. From the top of a tower, 80 m high from the ground, a stone is thrown in the horizontal direction with a velocity of 8 ms-1 . The stone reaches the ground after a time ‘t’ and falls at a distance of ‘d ’ from the foot of the tower.
Assuming g = 10 ms-2 , the time t and distance d are given respectively by
(A) 6 s, 64 m (B) 6 s, 48 m (C) 4 s, 32 m (D) 4 s, 16 m
14. A Wheatstone bridge has the resistances 10 Ω, 10 Ω, 10 Ω and 30 Ω in its four arms. What resistance joined in parallel to the 30 Ω resistance will bring it to the balanced condition ?
(A) 2 Ω (B) 5 Ω (C) 10 Ω (D) 15 Ω
15. An electric bulb marked as 50 W-200 V is connected across a 100 V supply. The present power of the bulb is
(A) 37.5 W (B) 25 W (C) 12.5 W (D) 10 W
16. In a mercury thermometer the ice point (lower fixed point) is marked as 10° and the steam point (upper fixed point) is marked as 130°. At 40°C temperature, what will this thermometer read ?
(A) 78° (B) 66° (C) 62° (D) 58°
17. The magnetic flux linked with a coil satisfies the relation Ø = 4t2 + 6t + 9 Wb, where t is the time in second. The e.m.f. induced in the coil at t = 2 second is
(A) 22V (B) 18V (C) 16V (D) 40V
18. Water is flowing through a very narrow tube. The velocity of water below which the flow remains a streamline flow is known as
(A) Relative velocity (B) Terminal velocity (C) Critical velocity (D) Particle velocity
19. If the velocity of light in vacuum is 3x108 ms-1 , the time taken (in nanosecond) to travel through a glass plate of thickness 10 cm and refractive index 1.5 is
(A) 0.5 (B) 1.0 (C) 2.0 (D) 3.0
20. A charge +q is placed at the origin O of X-Y axes as shown
in the figure. The work done in taking a charge Q from A to B along
the straight line AB is
(A) (B)
(C)
(D)
Q.21 What current will flow through the 2 kΩ resistor
in the circuit shown in the figure ?
(A) 3 mA (B) 6 mA (C) 12 mA (D) 36 mA
22. In a region, the intensity of an electric field is given by Ē = 2i +3j + k in NC -1 . The electric flux through a surface S = 10i m2 in the region is
(A) 5 Nm2C-1 (B) 10 Nm2C-1 (C) 15 Nm2C-1 (D) 20 Nm2C-1
23. The dimension of angular momentum is
(A) M0L1T-1 (B) M1L2T-2 (C) M1L2T-1 (D) M2L1T-2
24. If A = B + C and A, B , C have scalar magnitudes of 5, 4, 3 units respectively then the angle between A and C is
(A) cos-1 (3/5) (B) cos-1 (4/5) (C) tt/2 (D) sin-1(3/4)
25. A particle is travelling along a straight line OX. The distance x (in metres) of the particle from 0 at a time t is given by x = 37 + 27t - t3 where t is time in seconds. The distance of the particle from 0 it comes to rest is
(A) 81 m (B) 91 m (C) 101 m (D) 111 m
26. A particle is projected from the ground with a kinetic energy E at an angle of 60° the horizontal. Its kinetic energy at the highest point of its motion will be
(A) (B) E/2 (C) E/4 (D) E/8
27. A bullet on 30 cm into its target loses its velocity by 50% What additional distance will it penetrate into target before it comes
(A) 30 cm (B) 20 cm (C) 10 cm (D) 5 cm
28. When a spring is stretched 10 cm, the potential energy stored is E When the spring is stretched by 10 cm more, the potential energy stored in the spring becomes
(A) 2E (B) 4E (C) 6E (D) 10E
29. Average distance of the Earth from the Sun is L1 . If one year of the Earth = D days, one year of another planet whose average distance from the Sun is L2, will be
(A) days (B)
days (C)
days (D)
days
30. A spherical ball A of mass 4kg, moving along a straight line strikes another spherical ball B of mass 1 kg at rest. After the collision, A and B move with velocities V1 ms-1 and V2 ms-1 respectively making angles of 30o and 60o with respect to the original direction of motion of A. The ratio will be
(A) (B)
(C)
(D)
Q. 31 to Q. 40 carry two marks each.
31. When a certain metal surface is illuminated with light of frequency v , the stopping potential for photoelectric current is V0. When the same surface is illuminated by light of frequency , the stopping potential is
. The threshhold frequency for photoelectric emission is
(A) (B)
(C)
(D)
32. Three blocks of mass 4 kg, 2 kg, 1 kg respectively
are in contact on a frictionless table as shown in the figure.
If a force of 14 N is applied on the 4 kg block, the contact
force between the 4 kg and the 2 kg block will be
(A) 2 N (B) 6 N (C) 8 N (D) 14 N
33. Let L be the length and d be the diameter of cross section of a wire. Wires of the same material with different L and d are subjected to the same tension along the length of the wire. In which of the following cases, the extension of wire will be the maximum ?
(A) L = 200 cm, d = 0.5 mm (B) L = 300 cm, d = 1.0 mm
(C) L = 50 cm, d= 0.05 mm (D) L = 100 cm, d = 0.2 mm
34. An object placed in front of a concave mirror at a distance of x cm from the pole gives a 3 times magnified real image. If it is moved to a distance of (x+5) cm, the magnification of their image becomes 2 . The focal length of the mirror is
(A) 15 cm (B) 20 cm (C) 25 cm (D) 30 cm
35. 22320 cal of heat is supplied to 100 g of ice at 0°C. If the latent heat of fusion of ice is 80 cal g-1 and latent heat of vaporization of water is 540 cal g-1, the final amount of water thus obtained and its temperature respectively are
(A) 8 g, 100°C (B) 100 g, 90°C (C) 92 g, 100°C (D)82 g, 100°C
36. A progressive wave moving along x-axis is represented by . The wavelength (
) at which the maximum particle velocity is 3 times the wave velocity is
(A) (B)
(C)
(D)
37. Two radioactive substances A and B have decay constants 5 and
respectively. At t = 0, they have the same number of nuclei. The ratio of number of nuclei of A to that of B will be (1 / e)2 after a time interval of
(A) (B)
(C)
(D)
38. A magnetic needle is placed in a uniform magnetic field and is aligned with the field. The needle is now rotated by an angle of 60° and the work done is W. The torque on the magnetic needle at this position is0
(A) (B)
(C)
(D)
39. In the adjoining figure the potential difference
between X and Y is 60 V. The potential difference
between the points M and N will be
(A) 10 V (B) 15V (C) 20 V (D) 30 V
40. A body when fully immersed in a liquid of specific gravity 1.2 weighs 44 gwt. The same body when fully immersed in water weighs 50 gwt. The mass of the body is
(A) 36 g (B) 48 g (C) 64 g (D) 80 g
Subject: Chemistry
Q. 41 - Q. 70 carry one mark each.
41. Which of the following does not represent the mathematical expression for the Heisenberg uncertainty principle ?
(A) Δx.Δp ≥ h/(4π) (B) Δx.Δv ≥ h/(4π m) (C) ΔE.Δt ≥ h/(4π) (D) ΔE.Δx ≥ h/(4π)
42. The stable bivalency of Pb and trivalency of Bi is
(A) due to d contraction in Pb and Bi
(B) due to relativistic contraction of the 6s orbitals of Pb and Bi, leading to inert pair effect
(C) due to screening effect
(D) due to attainment of noble liquid configuration
43. The equivalent weight of K2Cr2O7 in acidic medium is expressed in terms of its molecular weight (M) as
(A) M/3 (B) M/4 (C) M/6 (D) M/7
44. Which of the following is correct ?
(A) radius of Ca2+ < CI- < S2- (B) radius of Cl- < 52- < Ca2+
(C) radius of S2- = CI- = Ca2+ (D) radius of S2- < CI- < Ca2+
45. CO is practically non-polar since
(A) the σ-electron drift from C to O is almost nullified by the π-electron drift from O to C
(B) the σ-electron drift from O to C is almost nullified by the π-electron drift from C to O
(C) the bond moment is low
(D) there is a triple bond between C and O
46. The number of acidic protons in H3PO3 are
(A) 0 (B) 1 (C) 2 (D) 3
47. When H2O2 is shaken with an acidified solution of K2Cr2O7 in presence of ether, the ethereal layer turns blue due to the formation of
(A) Cr2O3 (B) Cr042- (C) Cr2(SO4)3 (D) CrO5
48. The state of hybridization of the central atom and the number of lone pairs over the central atom in POCl3 are
(A) sp, 0 (B) sp2, 0 (C) sp3, 0 (D) dsp2, 1
49. Li occupies higher position in the electrochemical series of metals as compared to Cu since
(A) the standard reduction potential of Li+/Li is lower than that of Cu2/Cu
(B) the standard reduction potential of Cu2+/Cu is lower than that of Li+/Li
(C) the standard oxidation potential of Li/Li+ is lower than that of Cu/Cu2+
(D) Li is smaller in size as compared to Cu
50. 11Na24 is radioactive and it decays to
(A) 9F20 and α-particles (B) 13Al24 and positron (C) 11Na23 and neutron (D) 12Mg24 and
β-particles
51. The paramagnetic behavior of B2 is due to the presence of
(A) 2 unpaired electrons in πb MO
(B) 2 unpaired electrons in π* MO
(C) 2 unpaired electrons in σ* MO
(D) 2 unpaired electrons in σb MO
52. A 100 ml 0.1 (M) solution of ammonium acetate is diluted by adding 100 ml of water. The pH of the resulting solution will be (pKa of acetic acid is nearly equal to pKb of NH4OH)
(A) 4.9 (B) 5.0 (C) 7.0 (D) 10.0
53. In 2-butene, which one of the following statements is true ?
(A) C1-C2 bond is a sp3-sp3 σ-bond (B) C2-C3 bond is a sp3-sp2 σ-bond
(C) C1-C2 bond is a sp3-sp2 σ-bond (D) C1-C2 bond is a sp2-sp2 σ-bond
54. The well known compounds, (+) - lactic acid and (-) - lactic acid, have the same molecular formula, C3H6O3. The correct relationship between them is
(A) constitutional isomerism (B) geometrical isomerism (C) identicalness (D) optical isomerism
55. The stability of Me2C = CH2 is more than that of MeCH2CH = CH2 due to
(A) inductive effect of the Me group
(B) resonance effect of the Me group
(C) hyperconjugative effect of the Me group
(D) resonance as well as inductive effect of the Me group
56. Upon treatment with l2 and aqueous NaOH, which of the following compounds will form iodoform ?
(A) CH3CH2CH2CH2CHO (B) CH3CH2COCH2CH3 (C) CH3CH2CH2CH2CH2OH (D) CH3CH2CH2CH(OH)CH3
57. Upon treatment with Al(OEt)3 followed by usual reactions (work up), CH3CHO will produce
(A) only CH3COOCH2CH3 (B) a mixture of CH3COOH and EtOH
(C) only CH3COOH (D) only EtOH
58. Friedel-Craft’s reaction using MeCl and anhydrous AlCl3 will take place most efficiently with
(A) Benzene (B) Nitrobenzene (C) Acetophenone (D) Toluene
59. Which one of the following properties is exhibited by phenol ?
(A) It is soluble in aq. NaOH and evolves CO2 with aq. NaHCO3
(B) It is soluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
(C) It is not soluble in aq. NaOH but evolves CO2 with aq. NaHCO3
(D) It is insoluble in aq. NaOH and does not evolve CO2 with aq. NaHCO3
60. The basicity of aniline is weaker in comparison to that of methyl amine due to
(A) hyperconjugative effect of Me-group in MeNH2
(B) resonance effect of phenyl group in aniline
(C) lower molecular weight of methyl amine as compared to that of aniline
(D) resonance effect of -NH2 group in MeNH2
61. Under identical conditions, the SN1 reaction will occur most efficiently with
(A) tert-butyl chloride (B) 1-chlorobutane (C) 2-methyl-1-chloropropane (D) 2-chlorobutane
62. Identify the method by which Me3CCO2H can be prepared.
(A) Treating 1 mol of MeCOMe with 2 moles of MeMgl
(B) Treating 1 mol of MeCO2Me with 3 moles of MeMgl
(C) Treating 1 mol of MeCHO with 3 moles of MeMgl
(D) Treating 1 mol of dry ice with 1 mol of Me3CMgl
63. Which one of the following characteristics belongs to an electrophile ?
(A) It is any species having electron deficiency which reacts at an electron rich C-centre
(B) It is any species having electron enrichment, that reacts at an electron deficient C-centre
(C) It is cationic in nature
(D) It is anionic in nature
64. Which one of the following methods is used to prepare Me3COEt with a good yield ?
(A) Mixing EtONa with Me3CCl
(B) Mixing Me3CONa with EtCl
(C) Heating a mixture of (1:1) EtOH and Me3COH in presence of conc. H2SO4
(D) Treatment of Me3COH with EtMgI
65. 58.5 gm of NaCl and 180 gm of glucose were separately dissolved in 1000 ml of water. Identify the correct statement regarding the elevation of boiling point (b.p.) of the resulting solutions.
(A) NaCl solution will show higher elevation of b.p.
(B) Glucose solution will show higher elevation of b.p.
(C) Both the solutions will show equal elevation of b.p.
(D) The b.p. elevation will be shown by neither of the solutions
66. Equal weights of CH4 and H2 are mixed in an empty container at 25°C. The fraction of the total pressure exerted by H2 is
(A) 1/9 (B) 1/2 (C) 8/9 (D) 16/17
67. Which of the following will show a negative deviation from Raoult’s law ?
(A) Acetone-benzene (B) Acetone-ethanol (C) Benzene-methanol (D) Acetone-chloroform
68. In a reversible chemical reaction at equilibrium, if the concentration of any one of the reactants is doubled, then the equilibrium constant will
(A) also bedoubled (B) behalved (C) remains the same (D) becomes one-fourth
69. Identify the correct statement from the following in a chemical reaction.
(A) The entropy always increases
(B) The change in entropy along with suitable change in enthalpy decides the fate of a reaction
(C) The enthalpy always decreases
(D) Both the enthalpy and the entropy remain constant
70. Which one of the following is wrong about molecularity of a reaction ?
(A) It may be whole number or fractional
(B) It is calculated from reaction mechanism
(C) It is the number of molecules of the reactants taking part in a single step chemical reaction
(D) It is always equal to the order of elementary reaction
Q. 71 to Q. 80 carry two marks each.
71 . By passing excess Cl2(g) in boiling toluene, which one of the following compounds is exclusively formed ?
(A) (B)
(C)
(D)
72. An equimolar mixture of toluene and chlorobenzene is treated with a mixture of conc. H2SO4 and conc. HNO3. Indicate the correct statement from the following.
(A) p-nitrotoluene is formed in excess
(B) equimolar amounts of p-nitrotoluehe and p-nitrochlorobenzene are formed
(C) p-nitrochlorobenzene is formed in excess
(D) m-nitrochlorobehzene is formed in excess
73. Among the following carbocations : Ph2C+CH2Me (I), PhCH2CH2CH+Ph (II), Ph2CHCH+Me (III) and Ph2C(Me)CH2+ (IV), the order of stability is
(A) IV > II > I > III (B) I > II > III > IV (C) II > I > IV > III (D) I > IV > III > II
74. Which of the followings is correct ?
(A) Evaporation of water causes an increase in disorder of the system
(B) Melting of ice causes a decrease in randomness of the system
(C) Condensation of steam causes an increase in disorder of the system
(D) There is practically no change in the randomness of the system when water is evaporated
75. On passing ‘C’ Ampere of current for time ‘t’ sec through 1 litre of 2 (M) CuSO4 solution (atomic weight of Cu = 63.5), the amount ‘m’ of Cu (in gm) deposited on cathode will be
(A) m = Ct/(63.5 x 96500)
(B) m = Ct/(31.25 x 96500)
(C) m = (C x 96500)/(31.25 x t)
(D) m = (31.25 x C x t) / 96500
76. If the 1st ionization energy of H atom is 13.6 eV , then the 2nd ionization energy of He atom is
(A) 27.2 eV (B) 40.8 eV (C) 54.4 eV (D) 108.8 eV
77. The weight of oxalic acid that will be required to prepare a 1000 ml (N/20) solution is
(A) 126/100 gm (B) 63/40 gm (C) 63/20 gm (D) 126/20 gm
78. 20 ml 0.1 (N) acetic acid is mixed with 10 ml 0.1 (N) solution of NaOH. The pH of the resulting solution is (pKa of acetic acid is 4.74)
(A) 3.74 (B) 4.74 (C) 5.74 (D) 6.74
79. In the brown ring complex [Fe(H2O)5(NO)]SO4, nitric oxide behaves as
(A) NO+ (B) neutral NO molecule (C) NO- (D) NO2-
80. The most contributing tautomeric enol form of MeCOCH2CO2Et is
(A) CH2 = C(OH)CH2CO2Et
(B) MeC(OH) = CHCO2Et
(C) MeCOCH = C(OH)OEt
(D) CH2 = C(OH)CH = C(OH)OEt
***