WEST BENGAL JOINT ENTRANCE EXAM ,2010
PHYSICS AND CHEMISTRY
1. Experimental investigations show that the intensity of solar radiation is maximum for a wavelength 480 nm in the visible region. Estimate the surface temperature of sun. Given Wein’s constant b = 2.88 × 10–3 mK.
(A) 4000 K (B) 6000 K (C) 8000 K (D) 106 K
2. The temperature of an ideal gas is increased from 120 K to 480 K. If at 120 K, the root mean square speed of gas molecules is v, then at 480 K it will be
(A) 4v (B) 2v (C) v2 (D) v4
3. Two mirrors at an angle θ° produce 5 images of a point. The number of images produced when θ is decreased to θ° – 30° is
(A) 9 (B) 10 (C) 11 (D) 12
4. The radius of the light circle observed by a fish at a depth of 12 meter is (refractive index of water = 4/3)
(A) 36√7 (B) 36√7 (C) 36√5 (D) 4√5
5. In Young’s double slit experiment, the fringe width is β. If the entire arrangement is placed in a liquid of refractive index n, the fringe width becomes :
(A) nβ (B) βn+1 (C) βn−1 (D) βn
6. A plano-convex lens (ƒ = 20 cm) is silvered at plane surface. Now focal length will be :
(A) 20 cm (B) 40 cm (C) 30 cm (D) 10 cm
7. The light beams of intensities in the ratio of 9 : 1 are allowed to interfere. What will be the ratio of the intensities of maxima and minima ?
(A) 3 : 1 (B) 4 : 1 (C) 25 : 9 (D) 81 : 1
8. If x1 be the size of the magnified image and x2 the size of the diminished image in Lens Displacement Method, then the size of the object is :
(A) √x1x2 (B) x1x2 (C) x21x2 (D) x1x22
9. A point charge +q is placed at the centre of a cube of side L. The electric flux emerging from the cube is
(A) qε0 (B) Zero (C) 6qL2ε0 (D) q6L2ε0
10. In the figure below, the capacitance of each capacitor is 3 μF. The effective capacitance between A and B is :
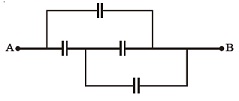
(A) 34μF (B) 3μF (C) 6μF (D) 5μF
11. n identical droplets are charged to v volt each. If they coalesce to form a single drop, then its potential will be
(A) n2/3v (B) n1/3v (C) nv (D) v/n
12. The reading of the ammeter in the following figure will be
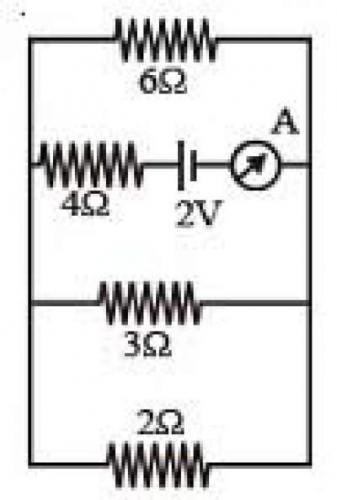
(A) 0.8 A (B) 0.6 A (C) 0.4 A (D) 0.2 A
13. A wire of resistance R is elongated n-fold to make a new uniform wire. The resistance of new wire
(A) nR (B) n2R (C) 2nR (D) 2n2R
14. The ratio of magnetic field and magnetic moment at the centre of a current carrying circular loop is x. When both the current and radius is doubled the ratio will be
(A) x/8 (B) x/4 (C) x/2 (D) 2x
15. The current through a coil of self inductance L = 2mH is given by I = t2e–t at time t. How long it will take to make the e.m.f. zero ?
(A) 1 s (B) 2 s (C) 3 s (D) 4 s
16. The magnetic flux through a loop of resistance 10 Ω is given by φ = 5t2 – 4t + 1 Weber. How muchcurrent is induced in the loop after 0.2 sec ?
(A) 0.4 A (B) 0.2 A (C) 0.04 A (D) 0.02 A
17. The decimal equivalent of the binary number (11010.101)2 is
(A) 9.625 (B) 25.265 (C) 26.625 (D) 26.265
18. In a common emitter configuration, a transistor has β = 50 and input resistance 1 kΩ. If the peak value of a.c. inputis 0.01 V then the peak value of collector current is
(A) 0.01 μA (B) 0.25 μA (C) 100 μA (D) 500 μA
19. Half-life of a radioactive substance is 20 minute. The time between 20% and 80% decay will be :
(A) 20 min (B) 30 min (C) 40 min (D) 25 min
20. The energy released by the fission of one uranium atom is 200 MeV. The number of fissions per second required to produce 3.2 W of power is (Take 1 eV = 1.6 × 10-19 J)
(A) 107 (B) 1010 (C) 1015 (D) 1011
21. A body is projected with a speed u m/s at an angle β with the horizontal. The kinetic energy at the highest point is 3/4th of the initial kinetic energy. The value of β is :
(A) 30° (B) 45° (C) 60° (D) 120°
22. A ball is projected horizontally with a velocity of 5 m/s from the top of a building 19.6 m high. How long will the ball take of hit the ground ?
(A) √2 s (B) 2 s (C) √3 s (D) 3 s
23. A stone falls freely from rest and the total distance covered by it in the last second of its motion equals the distance covered by it in the first three seconds of its motion. The stone remains in the air for
(A) 6 s (B) 5 s (C) 7 s (D) 4 s
24. Two blocks of 2 kg and 1 kg are in contact on a frictionless table. If a force of 3 N is applied on 2 kg block, then the force of contact between the two blocks will be :
![]()
(A) 0 N (B) 1 N (C) 2 N (D) 3 N
25. If momentum is increased by 20%, then kinetic energy increases by
(A) 48% (B) 44% (C) 40% (D) 36%
26. A boy of mass 40 kg is climbing a vertical pole at a constant speed. If the coefficient of friction between his palms and the pole is 0.8 and g = 10 m/s2, the horizontal force that he is applying on the pole is
(A) 300 N (B) 400 N (C) 500 N (D) 600 N
27. The value of ‘λ’ for which the two vectors →a=5ˆi+λˆj+ˆk and →b=ˆi−2ˆj+ˆk are perpendicular to each other is
(A) 2 (B) – 2 (C) 3 (D) – 3
28. If →a+→b=→c and a + b = c, then the angle included between →a and →b is
(A) 90° (B) 180° (C) 120° (D) Zero
29. The height vertically above the earth’s surface at which the acceleration due to gravity becomes 1% of its value atthe surface is (R is the radius of the Earth)
(A) 8 R (B) 9 R (C) 10 R (D) 20 R
30. The change in the gravitational potential energy when a body of mass m is raised to a height nR above the surface of the Earth is (here R is the radius of the Earth)
(A) (nn+1)mgR (B) (nn−1)mgR (C) nmgR (D) mgRn
31. A particle of mass m is attached to three identical massless springs of spring constant ‘k’ as shown in the figure. The time period of vertical oscillation of the particle is
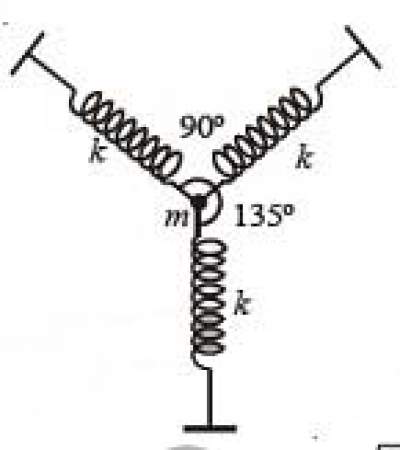
(A) 2π√mk (B) 2π√m2k (C) 2π√m3k (D) π√mk
32. A spring of force constant k is cut into three equal parts. The force constant of each part would be
(A) k3 (B) 3k (C) k (D) 2k
33. A body floats in water with 40% of its volume outside water. When the same body floats in oil, 60% of its volume remains outside oil. The relative density of the oil is
(A) 0.9 (B) 1.2 (C) 1.5 (D) 1.8
34. A uniform long tube is bent into a circle of radius R and it lies in vertical plane. Two liquids of same volume but densities ρ and δ fill half the tube. The angle θ is
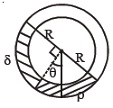
(A) tan−1(p−δp+δ) (B) tan−1pδ (C)tan−1δp (D) tan−1(p+δp−δ)
35. Two solid spheres of same metal but of mass M and 8 M fall simultaneously on a viscous liquid and their terminal velocities are v and nv then value of n is
(A) 16 (B) 8 (C) 4 (D) 2
36. A particle is executing linear simple harmonic motion of amplitude A. At what displacement is the energy of the particle half potential and half kinetic ?
(A) A4 (B) A2 (C) A√2 (D) A√3
37. The equation of a progressive wave is y = 4 sin (4πt – 0.04 x + π/3) where x is in meter and t is in second. The velocity of the wave is
(A) 100π m/s (B) 50π m/s (C) 25π m/s (D) π m/s
38. A longitudinal wave is represented by x = x0 sin 2π(nt – x/λ). The maximum particle velocity will befour times the wave velocity if :
(A) λ=πx04 (B) λ=2πx0 (C) λ=πx02 (D) λ=4πx0
39. A block of ice at temperature –20°C is slowly heated and converted to steam at 100°C. Which of the following diagram is most appropriate ?
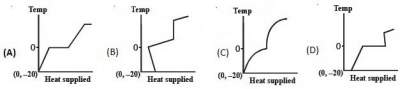
40. Two black bodies at temperatures 327°C and 427°C are kept in an evacuated chamber at 27°C. The ratio oftheir rates of loss of heat are :
(A) 67 (B) (67)2 (C) (67)3 (D) 243464
41. At identical temperature and pressure, the rate of diffusion of hydrogen gas is 3√3 times that of ahydrocarbon having molecular formula CnH2n–2 . What is the value of ‘n’ ?
(A) 1 (B) 4 (C) 3 (D) 8
42. Dipole moment of ![]() is 1.5D. The dipole moment of
is 1.5D. The dipole moment of ![]() is
is
(A) 1.5 D (B) 2.25 D (C) 1 D (D) 3 D
43. Which of the following thermodynamic relation is correct ?
(A) dG = VdP – SdT (B) dE = PdV + TdS (C) dH = – VdP + TdS (D) dG = VdP + SdT
44. In the hydrolysis of an organic chloride in presence of large excess of water; RCI + H2O → ROH +HCl
(A) Molecularity and order of reaction both are 2 (B) Molecularity is 2 but order of reaction is 1
(C) Molecularity is 1 but order of reaction is 2 (D) Molecularity is 1 and order of reaction is also 1
45. The potential of a hydrogen electrode at pH = 10 is
(A) 0.59 V (B) 0.00 V (C) –0.59 V (D) –0.059
46. Calculate Kc for the reversible process given below if Kp = 167 and T = 800°C
CaCO3(s)⇌CaO(s)+CO2(g)
(A) 1.95 (B) 1.85 (C) 1.89 (D) 1.60
47. For a reversible chemical reaction where the forward process is exothermic, which of the following statements is correct ?
(A) The backward reaction has higher activation energy than the forward reaction
(B) The backward and the forward processes have the same activation energy
(C) The backward reaction has lower activation energy
(D) No activation anergy is required at all since energy is liberated in the process.
48. In Sommerfeld’s modification of Bohr’s theory, the trajectory of an electron in a hydrogen atom is
(A) a perfect ellipse
(B) a closed ellipse – like curve, narrower at the perihelion position and flatter at the aphelion position
(C) a closed loop on spherical surface
(D) a rosette
49. In the reaction of sodium thiosulphate with I2 in aqueous medium the equivalent weight of sodium thiosulphate is equal to
(A) molar mass of sodium thiosulphate
(B) the average of molar masses of Na2S2O3 and I2
(C) half the molar mass of sodium thiosulphate
(D) molar mass of sodium thiosulphate × 2
50. 0.1 (M) HCI and 0.1 (M) H2SO4 each of volume 2ml are mixed and the volume is madeup to 6 ml by adding 2ml of 0.01 (N) NaCl solution. The pH of the resulting mixture is
(A) 1.17 (B) 1.0 (C) 0.3 (D) log 2 – log 3
51. The molarity of a NaOH solution by dissolving 4 g of it in 250 ml water is
(A) 0.4 M (B) 0.8 M (C) 0.2 M (D) 0.1 M
52. If a species has 16 protons, 18 electrons and 16 neutrons, find the species and its charge
(A) S1– (B) Si2– (C) P3– (D) S2–
53. In a periodic table the basic character of oxides
(A) increases from left to right and decreases from top to bottom
(B) decreases from right to left and increases from top to bottom
(C) decreases from left to right and increases from top to bottom
(D) decreases from left to right and increases from bottom to top
54. Which one of the following contains P – O – P bond ?
(A) Hypophosphorus acid (B) Phosphorus acid (C) Pyrophosphoric acid (D) Orthophosphoric acid
55. Which of the following orders regarding ionization energy is correct ?
(A) N > O > F (B) N < O < F (C) N > O < F (D) N < O > F
56. Which of the following statements regarding ozone is not correct ?
(A) The Ozone molecule is angular in shape
(B) The Ozone is a resonance hybrid of two structures
(C) The Oxygen–Oxygen bond length in ozone is identical with that of molecular oxygen
(D) Ozone is used as germicide and disinfectant for the purification of air
57. P4O10 is the anhydride of
(A) H3PO2 (B) H3PO3 (C) H3PO4 (D) H4P2O7
58. Which of the following metals has the largest abundance in the earth’s crust ?
(A) Aluminium (B) Calcium (C) Magnesium (D) Sodium
59. Which of the following orbitals will have zero probability of finding the electron in the yz plane ?
(A) Px (B) Py (C) Pz (D) dyz
60. What type of orbital hybridisation is considered on P in PCl5 ?
(A) sp3d (B) dsp3 (C) sp3d2 (D) d2sp3
61. For which element the inertness of the electron pair will not be observed ?
(A) Sn (B) Fe (C) Pb (D) In
62. In which of the following molecules is hydrogen bridge bond present ?
(A) Water (B) Inorganic benzene (C) Diborane (D) Methanol
63. When a manganous salt is fused with a mixture of KNO3 and solid NaOH the oxidation number of Mnchanges from +2 to
(A) +4 (B) +3 (C) +6 (D) +7
64. In hemoglobin the metal ion present is
(A) Fe2+ (B) Zn2+ (C) Co2+ (D) Cu2+
65. Ortho-and para-hydrogens have
(A) Identical chemical properties but different physical properties
(B) Identical physical and chemical properties
(C) Identical physical properties but different chemical properties
(D) Different physical and chemical properties
66. The bond order of CO molecule is
(A) 2 (B) 2.5 (C) 3 (D) 3.5
67. Vitamin C is
(A) Citric acid (B) Lactic acid (C) Paracetamol (D) Ascorbic acid
68. On mixing an alkane with chlorine and irradiating with ultra-violet light, it forms only one mono-chloro-alkane. The alkane is
(A) Propane (B) Pentane (C) Isopentane (D) Neopentane
69. Keto-enol tautomerism is not observed in
(A) C6H5COC6H5 (B)C6H5COCH=CH2 (C)C6H5COCH2COCH3 (D)CH3COCH2COCH3
70. What is obtained when nitrobenzene is treated sequentially with (i) NH4Cl/Zn dust and (ii) H2SO4/Na2Cr2O7 ?
(A) meta-chloronitrobenzene (B) para-chloronitrobenzene (C) nitrosobenzene (D) benzene
71. Boiling water reacts with C6H5N2+Cl- to give
(A) aniline (B) benzylamine (C) phenol (D) benzaldehyde
72. Aspirin is
(A) Acetyl salicylic acid (B) Benzoyl salicylic acid (C) Chloro benzoic acid (D) Anthranilic acid
73. XPCl5⟶C2H5Cl
YPCl5⟶CH3COCl
X and Y are
(A) (C2H5)2O and CH3CO2H (B)C2H5I and C2H5CHO (C) C2H5OHand CH3CO2H (D) C2H5OH andC2H5CHO
74. Which of the following compounds shows evidence of the strongest hydrogen bonding ?
(A) Propan–1–ol (B) Propan–2–ol (C) Propan–1,2–diol (D) Propan–1,2,3–triol
75. When AgCl is treated with KCN
(A) Ag is precipitated (B) a complex ion is formed (C) double decomposition takes place (D) no reaction takes place
76. Which one of the following produced when acetone is saturated with HCl gas ?
(A) Acetone alcohol (B) Phorone (C) Mesityl oxide (D) Benzene
77. Which one of the following is an example of co-polymer ?
(A) Buna–S (B) Teflon (C) PVC (D) Polypropylene
78. Identify [A] and [B] in the following
22789Ac−β⟶[A]−α⟶[B]−α⟶Rn
(A) Po, Rn (B) Th, Po (C) Ra, Th (D) Th, Ra
79. A weak acid of dissociation constant 10–5 is being titrated with aqueous NaOH solution. The pH atthe point of one-third neutralisation of the acid will be
(A) 5 + log 2–log 3 (B) 5 –log 2 (C) 5 –log 3 (D) 5 –log 6
80. Radioactivity of a sample (z=22) decreases 90% after 10 years. What will be the half life of the sample ?
(A) 5 years (B) 2 years (C) 3 years (D) 10 years
***






