Madhyamik Examination (WBBSE) - 2018 Physical Science (Eng ver)
GROUP—A1
1. Multiple choice questions: 1x15=15
Four alternatives are given as answer for each of the following questions. Write the correct one :
1.1 Which of the following greenhouse gases has maximum contribution towards global warming ?
(a) N2O (b) CH4 (c) CO2 (d) H2O vapour
1.2 According to Boyle's law which is the PV-P graph—
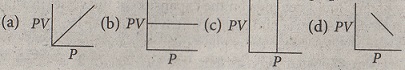
1.3 If the vapour density of a carbon containing gaseous substance is 13, which of the following can be its molecular formula ?
(a) CO2 (b) C2H4 (c) C2H6 (d) C2H2
1.4 The unit of co-efficient of linear expansion of a solid is—
(a) m (b) m-1 (c) °C-1 (d) °C
1.5 An object is placed in between the optical centre and focus of a thin convex lens. What is the nature of image of the object ? —
(a) real and inverted (b) virtual and inverted (c) real and erect (d) virtual and erect
1.6 When a ray of light is incident perpendicularly on a transparent glass slab—
(a) 0° (b) 180° (c) 30° (d) 90°
1.7 Which of the units given below is the SI unit of resistance ?
(a) volt (b) ampere (c) coulomb (d) ohm
1.8 In domestic electric circuit the fuse wire is connected to which of the following ?
(a) earth line (b) live line (c) neutral line (d) both live and neutral line
1.9 ß-ray emitted from a radioactive element is—
(a) a stream of electrons (b) a stream of protons (c) a stream of neutrons (d) electromagnetic wave
1.10 How many groups are there in the long periodic table ?
(a) 7 (b) 8 (c) 9 ( d) 18
1.11 In formation of which of the following compounds octet rule is not obeyed ?
(a) NaCl (b) LiH (c) KCl (d) CaO
1.12 Which of the following can conduct electricity ?
(a) molten NaCl (b) liquid HCl (c) solid NaCl (d) aqueous solution of glucose
1.13 What will be the colour of the resulting solution when excess aqueous ammonia is added to an aqueous solution of copper sulphate ?
(a) yellow (b) green (c) deep blue (d) brown
1.14 In which of the following alloys zinc is present ?
(a) bell metal (b) brass (c) bronze (d) duralumin
1.15 Which of the following is a saturated hydrocarbon ?
(a) C3H6 (b) C2H4 (c) C2H2 (d) C2H6
GROUP—B
2. Answer the following questions (alternatives are to be noted) : 1x21=21
2.1 Mention one use of bio-gas. Or, What is the role of NO in decomposition of ozone in the ozone layer ?
2.2 Among charcoal, petrol and ethanol which one is a fossil fuel ?
2.3 Under constant pressure, at what temperature in degree Celsius the volume of an Ideal gas will be zero according to Charles's Law ?
2.4 What is the unit of M in the equation PV=WMRT ? (symbols have usual meaning)
2.5 Whether the following statement is 'True' or 'False' ?
The real expansion of any liquid depends on the expansion of the vessel in which it is kept. Or, Among iron, invar and copper which one has the least co-efficient of linear expansion ?
2.6 Between the angle of incidence and the angle of refraction which one is greater when light travels from a rarer to a denser medium ?
2.7 What type of mirror is used in the viewfinder of a motor car ?
2.8 How does the resistance of a semiconductor change with increase of temperature ?
2.9 Which type of energy is transformed to electrical energy in a dynamo ?
2.10 Arrange α, ß and γ-rays in ascending order of their penetrating power. Or, Which kind of nuclear reaction is the source of sun's energy ?
2.11 Match the left column with the right column : 1x4=4
| Left Column | Right Column | ||
| 2.11.1 | An alkali metal | (i) | F |
| 2.11.2 | An element whose anion accelerates rusting of iron | (ii) | Fe |
| 2.11.3 | Extracted from hematite | (iii) | K |
| 2.11.4 | Most electronegative element | (iv) | Cl |
2.12 What type of chemical bond is present in CaO ?
2.13 What is used as cathode to electroplate silver over a copper spoon ? Or, Give example of a compound whose aqueous solution is a weak electrolyte.
2.14 During electrolysis which electrode is called a cathode ?
2.15 State one use of liquid ammonia. Or, Write the formula of the precipitate formed when aqueous ammonia solution is added to aqueous solution of aluminium chloride
2.16 In the laboratory preparation of nitrogen, aqueous solution of which compound is mixed with aqueous solution of ammonium chloride and heated ?
2.17 Write the IUPAC name of CH3CH2CHO. Or, Write the structural formula of positional isomer of CH3CH2CHO.
2.18 Mention one use of poly(tetrafluoroethylene).
GROUP—C
3. Answer the following questions (alternatives are to be noted): 2x9=18
3.1 What is methane hydrate ?
3.2 The pressure of a fixed mass of a gas at a temperature of 0°C is doubled while the volume is halved. What will be the final temperature of the gas ? Or, Under constant pressure a fixed mass of a gas is heated from 0°C to 546°C. What is the ratio of the final volume of the gas with its initial volume ?
3.3 What is meant by the optical center of a convex lens ? Or, Why does the earth's sky appear blue during day time ?
3.4 State Lenz's Law related to electromagnetic induction.
3.5 Write with an example how according to Lewis concept a covalent bond is formed. Or, Why the bond in sodium chloride cannot be expressed as Na—Cl ?
3.6 Give one example each of a liquid and a solid covalent compound ?
3.7 Write with balanced chemical equation what happens when H2S gas is passed through an aqueous copper sulphate solution.
3.8 Write down cathode reaction when an aqueous solution of MSO4 (M = metal) is electrolysed.
Write with reason whether the reaction is oxidation or reduction. Or, Give one use of each of copper and aluminium.
3.9 What is the condition of substitution reaction of methane with chlorine ? Write the balanced chemical equation of the first step of the reaction. Or, Write with balanced chemical equation what happens when ethanol reacts with metallic sodium.
GROUP—D
4. Answer the following questions (alternatives are to be noted): 3x12=36
4.1 Establish ideal gas equation on the basis of Boyle's Law, Charles's Law and Avogadro's Law.
4.2 SO2 required for the industrial production of sulphuric acid is produced by burning iron pyrites in excess air current.
The chemical equation of the reaction is given below :
4FeS2 + 11O2 → 2Fe2O3 + 8SO2. How many gram of FeS2 is required for production of 512 g of SO2 ? [Fe = 56, S = 32, O = 16] Or, By heating 200 g of a metal carbonate 112 g metal oxide and a gaseous compound are produced. Vapour density of the gaseous compound is 22. How many moles of the gaseous compound is produced in the reaction ?
4.3 What is thermal conductivity ? What is its SI unit ? (2+1) Or, Define co-efficient of surface expansion. Write its SI unit ? (2+1)
4.4 How can an erect and magnified image be formed with the help of a convex lens ? With the help of which type of lens long-sightedness can be rectified ? (2+1)
4.5 If the velocity of light in a medium is 2 x 108 m/s, what will be the refractive index of that medium. Or, The refractive index of a medium with respect to air is √2. If the angle of incidence of a ray of light in air is 45° determine the angle of deviation for that ray in case of refraction.
4.6 Write Joule's Laws related to heating effect of current.
4.7 Calculate the equivalent resistance when a wire of resistance 10 ohm is divided in two equal parts and connected in parallel comblnatlon. Or, There are two 60 watt lamps and two 80 watt fans in a house. The lamps and fans run 5 hours daily. Find out the expense in a month if an unit of electricity costs Rs.4/-. (assume one month = 30 days)
4.8 Compare the charge and ionising power of α and γ-rays. Mention one use of radioactivity. (2+1)
4.9 What is meant by ionisation energy of an atom of an element ? Arrange Li, Rb, K and Na in the increasing order of their ionisation. (2+1) Or, Mention similarity of properties of hydrogen with one property of Group 1 elements and two properties of Group 17 elements. (2+1)
4.10 What are present along with pure alumina in the molten mixture which is electrolysed for the extraction of aluminium by electrolysis ? What are used as cathode and anode in this electrolysis ? (1+2)
4.11 Write the conditions and balanced chemical equation for the industrial production of ammonia by Haber's process.
4.12 The molecular formula of an organic compound is C2H4O2. The compound is soluble in water and on addition of NaHCO3 to the aqueous solution of the compound CO2 is evolved. Identify the organic compound. Write with conditions and balanced chemical equation, the reaction of the compound with ethanol. (1+2) Or, Compare three properties of organic and inorganic compounds.
*****






